EXINI executes agreement with INmune Bio for Phase I/II trial
CaRe PC is an open label Phase I/II trial that will test up to three doses of INKmune™ in men with mCRPC. INKmune™ is given as out-patient therapy via intravenous infusion three times in the first two weeks of treatment (days 1, 8 and 15). The patient is followed for...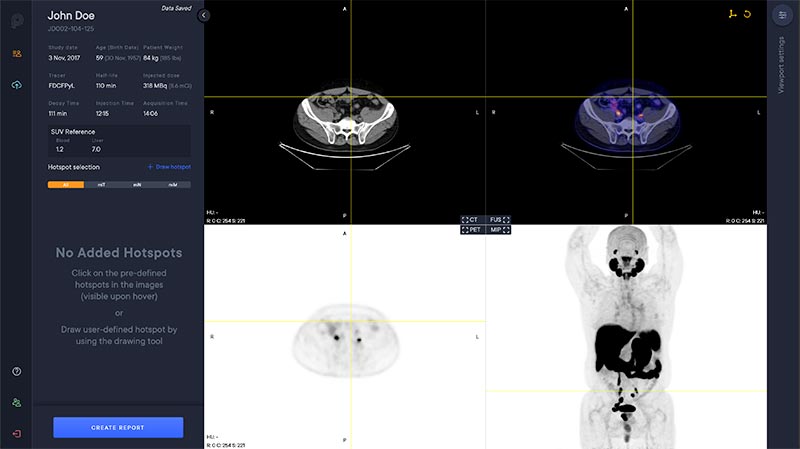
New version released of PYLARIFY AI® (aPROMISE)
We have now released a new version of PYLARIFY AI® for prostate cancer analysis. The new version include features such as: Parallel Study Review Select all suggested hotspots CT Measurement Tool Configurable Crosshair User Specific Settings Report Improvements...Lantheus Announces Top Rated Oral Presentation Featuring PYLARIFY AI® at the 2023 EuropeanAssociation of Nuclear Medicine (EANM) Annual Meeting
PYLARIFY AI™ (aPROMISE) is the only deep learning enabled FDA-cleared medical device software to offer standardized PSMA PET reporting on PSMA PET/CT images, including those achieved using PYLARIFY® (piflufolastat F18) PET/CT.1 Standardized PSMA PET reporting provides...EXINI Diagnostics AB, a Lantheus company (EXINI), announced today a ‘License and Development’ agreement with Curium, a world leader in nuclear medicine , to customize EXINI’s artificial intelligence (AI) platform for use in Europe.
Under the terms of the agreement, EXINI’s deep learning platform will be tailored to meet the specific needs of the European market. The clinical value proposition of the platform is to increase the efficiency and reproducibility of PSMA PET/CT image assessments in...Lantheus Announces Presentations Featuring PYLARIFY AI® at the 2023 Society for NuclearMedicine and Molecular Imaging (SNMMI) Annual Meeting
PYLARIFY AI™ (aPROMISE) is the only deep learning enabled FDA-cleared medical device software to offer standardized PSMA PET reporting with PYLARIFY AI on PSMA PET/CT images, including those achieved using PYLARIFY® (piflufolastat F18) PET/CT.1 Standardized PSMA PET...
EXINI Executes Agreement with Siemens Healthineers for the Distribution of its PSMA Artificial Intelligence Technology aPROMISE and quantitative assessment technology of bone scans aBSI
As of November 2022, EXINI has entered into an agreement with Siemens Healthineers to integrate its artificial intelligence (AI) enabled software as a medical devices – aPROMISE™ (automated PROstate Cancer Molecular Imaging Standardized Evaluation) and aBSI®...
EXINI Executes Agreement with Bayer AG for the Distribution of its PSMA Artificial Intelligence Technology aPROMISE and quantitative assessment technology of bone scans aBSI
As of November 2022, EXINI has entered into an agreement with Bayer AG to integrate its artificial intelligence (AI) enabled software as a medical devices – aPROMISE™ (automated PROstate Cancer Molecular Imaging Standardized Evaluation) and aBSI® (Automated Bone Scan...
