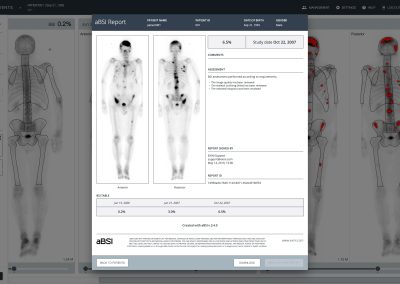
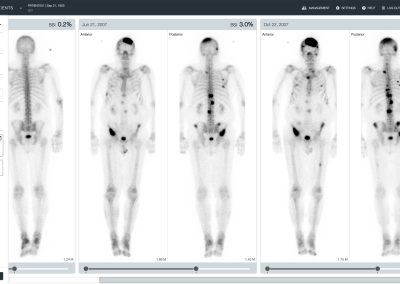
aBSI – Quantification of Bone Scans
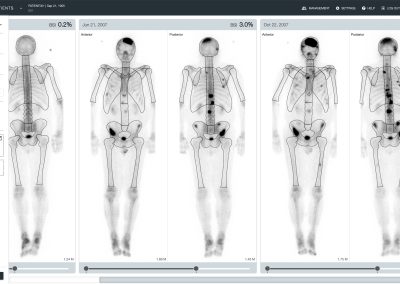
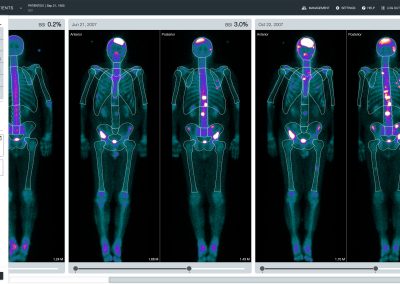
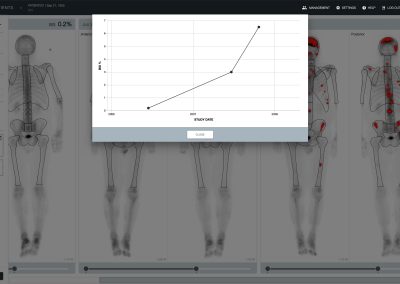
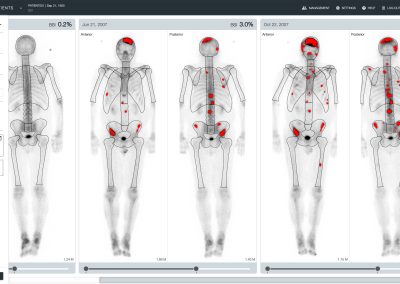
The automated Bone Scan Index (aBSI) technology gives a fully quantitative assessment of bone scans that incorporates inferred masses of all lesions. The computed index reflects the proportion of the total skeleton mass that is observed to have tumor involvement1.
In a prospectively defined multi-institutional phase 3 study with 721 metastatic prostate cancer patients, aBSI was found to be an independent prognostic determinant of overall survival and the study supported using aBSI in the design and eligibility for clinical trials for systemic therapies for metastatic castration-resistant prostate cancer2.
aBSI is also available for local installation under the name EXINI bone.
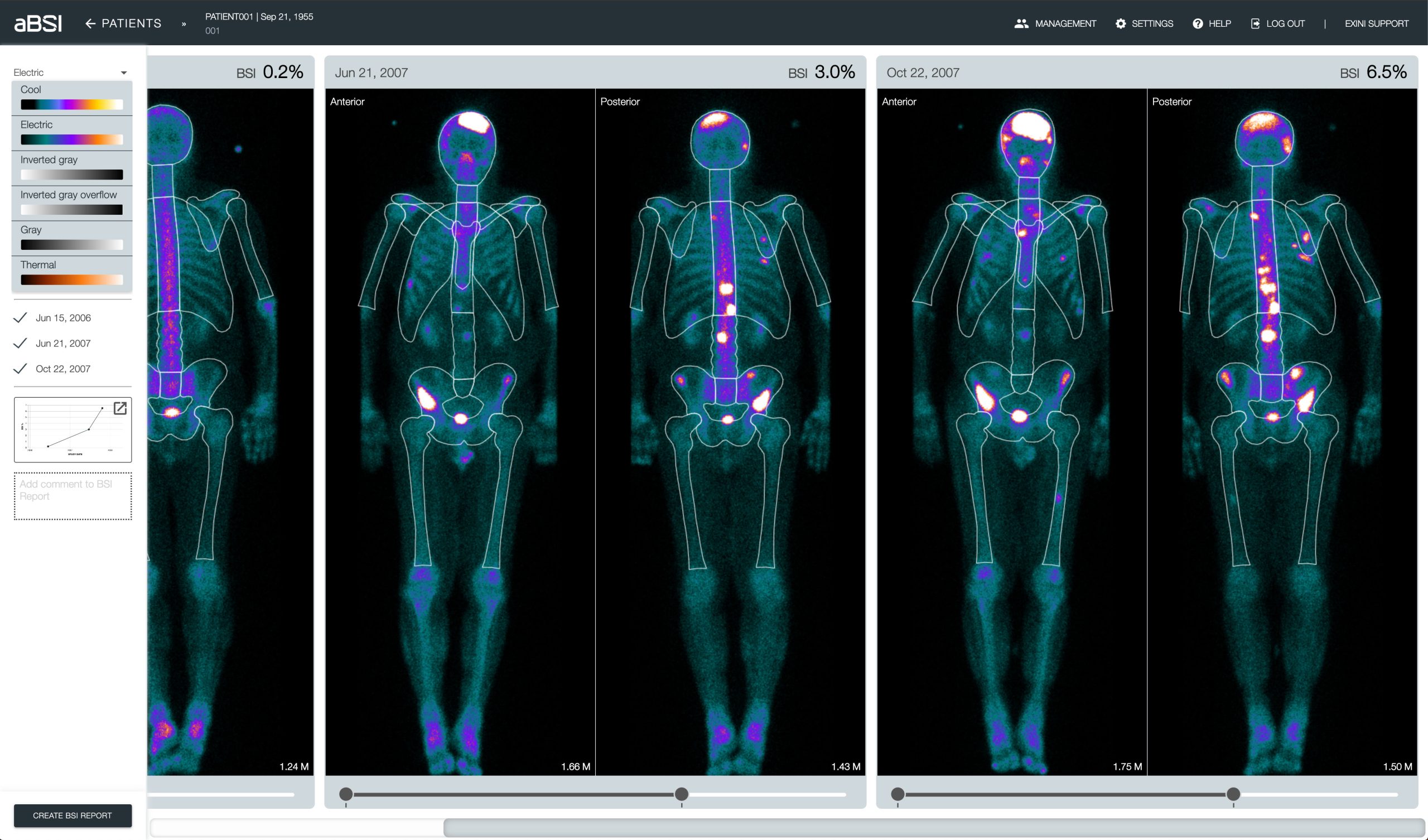
aBSI assessments3
- Accurate
- Quantitative
- Reproducible
Explore aBSI
Learn more about the advantages of Bone Scan Index
Use aBSI
Login
If you already have an account, you can use it to log in to aBSI from your computer.
Publications
Our technology, included in our product aBSI and BONENAVI, is well documented in several studies.
Help
Contact information to our customer support.
Performance and safety
Performance and safety for aBSI and EXINI bone
Legal
References
- Ulmert D, et al. A novel automated platform for quantifying the extent of skeletal tumour involvement in prostate cancer patients using the Bone Scan Index. Eur Urol. 2012.
- Armstrong AJ et al. Phase 3 Assessment of the Automated Bone Scan Index as a Prognostic Imaging Biomarker of Overall Survival in Men With Metastatic Castration-Resistant Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018.
- Anand A, et al. Journal of Nuclear Med. 2016 Jan;57(1):41-5.